Iridium Single Atoms to Nanoparticles: Nurturing the Local Synergy with Cobalt-Oxide Supported Palladium Nanoparticles for Oxygen Reduction Reaction
In brief, the present work not only contributes to a highly efficient and economically competitive ORR catalyst but also provides the fundamental understanding of structure–performance relationship and thus offers advancements in scientific and industrial developments for ORR catalysis.
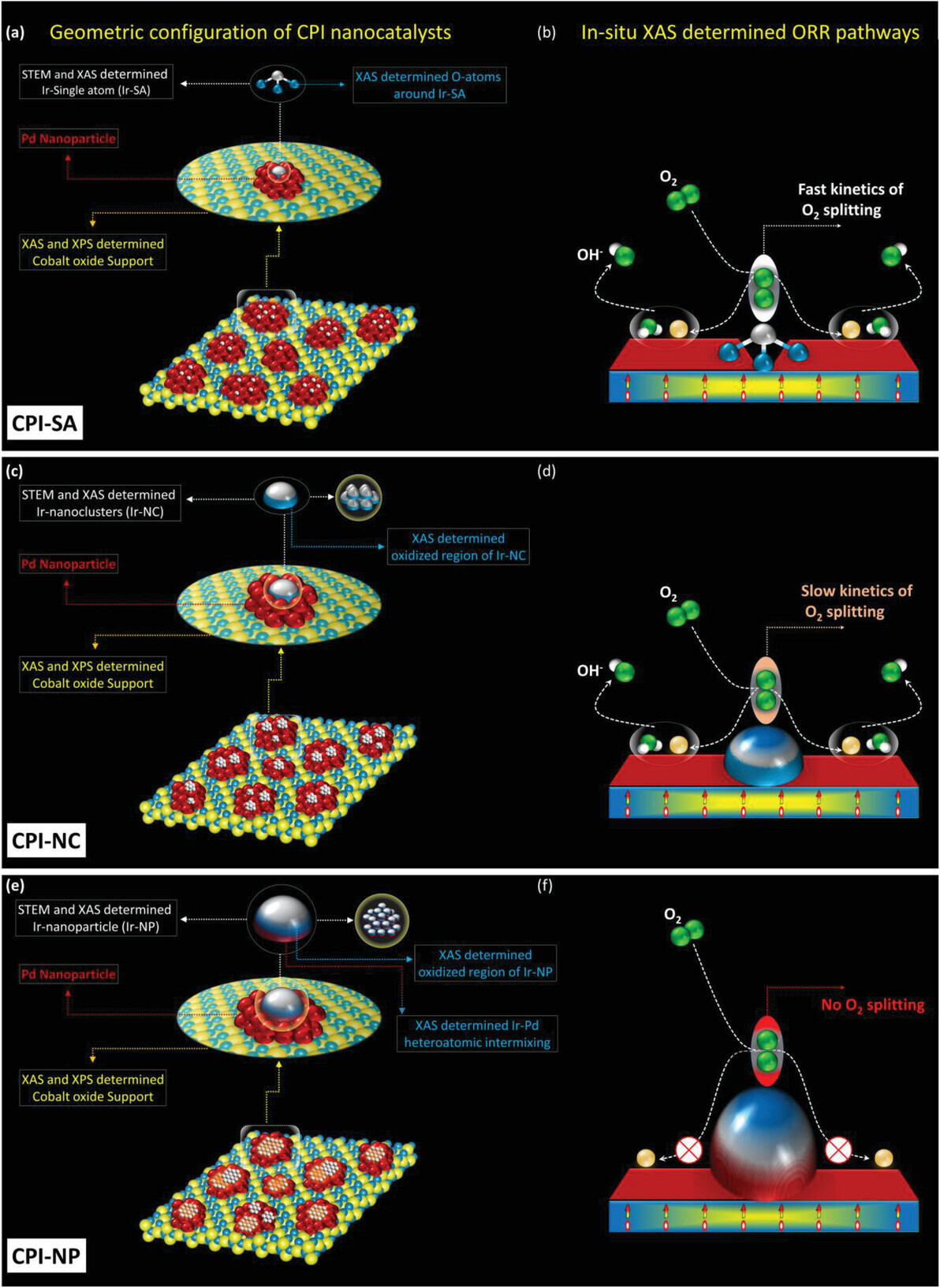
Figure 6. The schematic geometric configuration of a) CPI-SA, c) CPI-NC, e) CPI-NP and corresponding ORR pathways of b) CPI-SA, d) CPI-NC, f) CPI-NP.
Technology Overview
This research addresses the limitations of single-atom catalysts (SACs) in fuel cells, particularly their low metal content, susceptibility to aggregation, and lack of ensemble sites, which hinder the crucial oxygen reduction reaction (ORR). The study proposes a novel approach: a ternary catalyst system combining Ir single atoms (Ir-SAs), Ir nanoclusters (Ir-NCs), and Ir nanoparticles (Ir-NPs). The optimal catalyst, cobalt-oxide-supported Pd NPs with surface-decorated Ir-SAs (CPI-SA), leverages a synergistic interaction where Ir-SAs boost O2 splitting and Pd NPs promote OH- relocation, significantly enhancing ORR performance.
Applications & Benefits
This breakthrough offers a highly efficient and durable electrocatalyst for fuel cells, crucial for their widespread adoption as a clean energy source. The CPI-SA catalyst demonstrates 107-fold higher mass activity than commercial Pt/C catalysts at 0.85V vs RHE and unprecedented durability, retaining 186% of its initial performance after 69,000 cycles. This advancement not only contributes to more cost-effective and long-lasting fuel cells but also provides fundamental insights into catalyst design, guiding the development of superior multi-component catalytic systems for various industrial applications.
Abstract:
A ternary catalyst comprising Iridium (Ir) single-atoms (SA)s decorated on the Co-oxide supported palladium (Pd) nanoparticles (denoted as CPI-SA) is developed in this work. The CPI-SA with 1 wt.% of Ir exhibits unprecedented high mass activity (MA) of 7173 and 770 mA mgIr−1, respectively, at 0.85 and 0.90 V versus RHE in alkaline ORR (0.1 m KOH), outperforming the commercial Johnson Matthey Pt catalyst (J.M.-Pt/C; 20 wt.% Pt) by 107-folds. More importantly, the high structural reliability of the Ir single-atoms endows the CPI-SA with outstanding durability, where it shows progressively increasing MA of 13 342 and 1372 mA mgIr−1, respectively, at 0.85 and 0.90 V versus RHE up to 69 000 cycles (3 months) in the accelerated degradation test (ADT). Evidence from the in situ partial fluorescence yield X-ray absorption spectroscopy (PFY-XAS) and the electrochemical analysis indicate that the Ir single-atoms and adjacent Pd domains synergistically promote the O2 splitting and subsequent desorption of hydroxide ions (OH−), respectively. Whereas the Co-atoms underneath serve as electron injectors to boost the ORR activity of the Ir single-atoms. Besides, a progressive and sharp drop in the ORR performance is observed when Ir-clusters and Ir nanoparticles are decorated on the Co-oxide-supported Pd nanoparticles.

Iridium Single Atoms to Nanoparticles: Nurturing the Local Synergy with Cobalt-Oxide Supported Palladium Nanoparticles for Oxygen Reduction Reaction
Author:Dinesh Bhalothia, Che Yan, Nozomu Hiraoka, Hirofumi Ishii, Yen‑Fa Liao, Sheng Dai, Po-Chun Chen, Tsan-Yao Chen
Year:2024
Source publication:Advanced Science, Volume11, Issue33, September 4, 2024
Subfield Highest percentage:99% Biochemistry, Genetics and Molecular Biology (miscellaneous) #1 / 125
https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202404076